Revalidation: How the transition works
19 Mar . 3 min read.
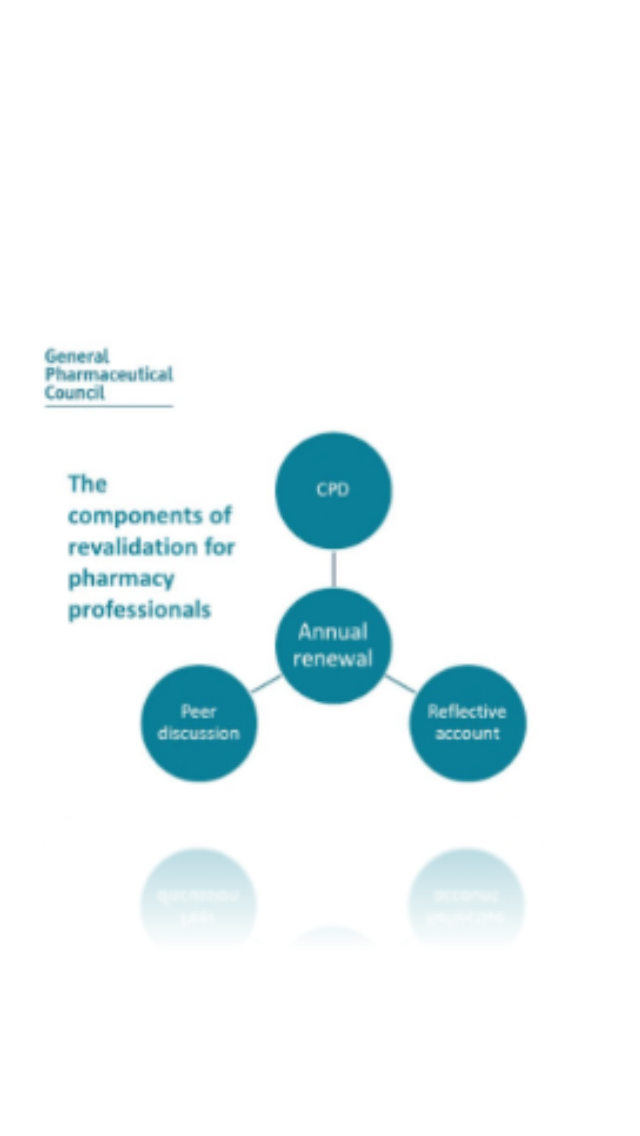
Your revalidation process is changing, are you aware what this change means for you? Do you fully understand why this is happening & what it requires you to do as a registered pharmacy professional?
We have prepared a bitesize explanation of the timeline to help you digest this information easily.
These changes to revalidation will be fully complete by September of 2020. After that every pharmacist will be required yearly to comply completely with the new requirements. The new requirements are as follows [1]:
● 4 CPD entries
2 From a planned learning event
2 From an unplanned learning event
● 1 Reflective essay
● 1 Peer discussion
We will discuss these individual requirements in detail in a later article, as the focus for this article is the timeline of this process and the new changes being implemented. The first cohort of pharmacists that will be required to comply with the new changes is any pharmacist whose revalidation date is the 31st of December 2018, that according to the PJ is around 54% of the profession [2].
In the first year of these new changes only 4 CPD entries are required. These must be uploaded to the new and updated MyGPhC website that goes live on the 30th of March 2018 [3]. These CPD entries need to officially be uploaded between the 1st of September and the 31st of October, two months before the revalidation deadline [2 & 3].
We stated ‘officially uploaded’ because you can upload your CPD entries to the new MyGPhC website any time after it goes live but they must be officially submitted when the revalidation time window opens. After the first group, remaining members renewal dates are either the 1st or 15th of every subsequent month [3]. The submission of the CPDs must be at least two months before their revalidation deadline for every registered pharmacy professional.
The following example can clarify this: Anyone whose revalidation is the 1st of August 2019 will have to submit 4 CPD entries between the 1st of April 2019 and the 31st of May 2019. The following year they will have to submit 4 CPD entries, a reflective account, and a peer discussion between the 1st of April 2020 and the 31st of May 2020 [4].
Starting December 2018, the new regulation will come into effect and this will be defined as the first cohort. Therefore, if your revalidation is the 1st of November 2018 then you use the current system and complete the declaration as you have been doing in previous years to revalidate. This involves logging onto MyGPhC website two months before the revalidation deadline, i.e. the 31st of August. Undertake the declaration that you have completed the required 9 CPD entries and pay the revalidation fee as normal [4]. The following year in 2019 the 4 submitted CPD entries would be required, between the 1st of July and 31st of August to be submitted. Then in 2020 the 4 CPD entries, reflective account, and peer discussion would be required [3].
These uploaded documents will not all be reviewed yearly, a sample of randomly selected submissions and a proportion of targeted professionals will have their submissions reviewed.
The GPhC have not assigned a figure on the number of randomly selected and targeted reviews that will be undertaken each year the PJ stated that “at least 1 in 40 registrants (2.5%) will be selected to have their records reviewed. Another 300–400 people will be specifically targeted”. You can be a targeted for review, for among other things: [2]
● “if they have required you previously to undertake remedial measures following a review of your records
● if you have a history of poor compliance with any of the standards, or
● if your records are submitted late without a good reason”
The review will be undertaken by two people, one will be a pharmacy professional and the other will be a lay person. You will be informed if your documents have been selected for renewal by email and told how long to wait until you know the outcome of the review.
Article by: Harry Cotterill in collaboration with Abbas Samnani
References:
1. https://www.pharmacyregulation.org/revalidation-glance, last accessed 12th March 2018
2. Revalidation: support and implementation. The Pharmaceutical Journal, 24 Jan 2018, By Ingrid Torjesen, https://www.pharmaceutical-journal.com/news-and-analysis/features/revalidation-support-and-implementation/20204267.article Last Accessed 12th March 2018
3. Revalidation Framework, January 2018. General Pharmaceutical Council 2018. https://www.pharmacyregulation.org/sites/default/files/document/gphc_revalidation_framework_january_2018.pdf, Last Accessed 12th March 2018
4. Personal Correspondence with the GPhC 3rd March 2018
